BROCHURE
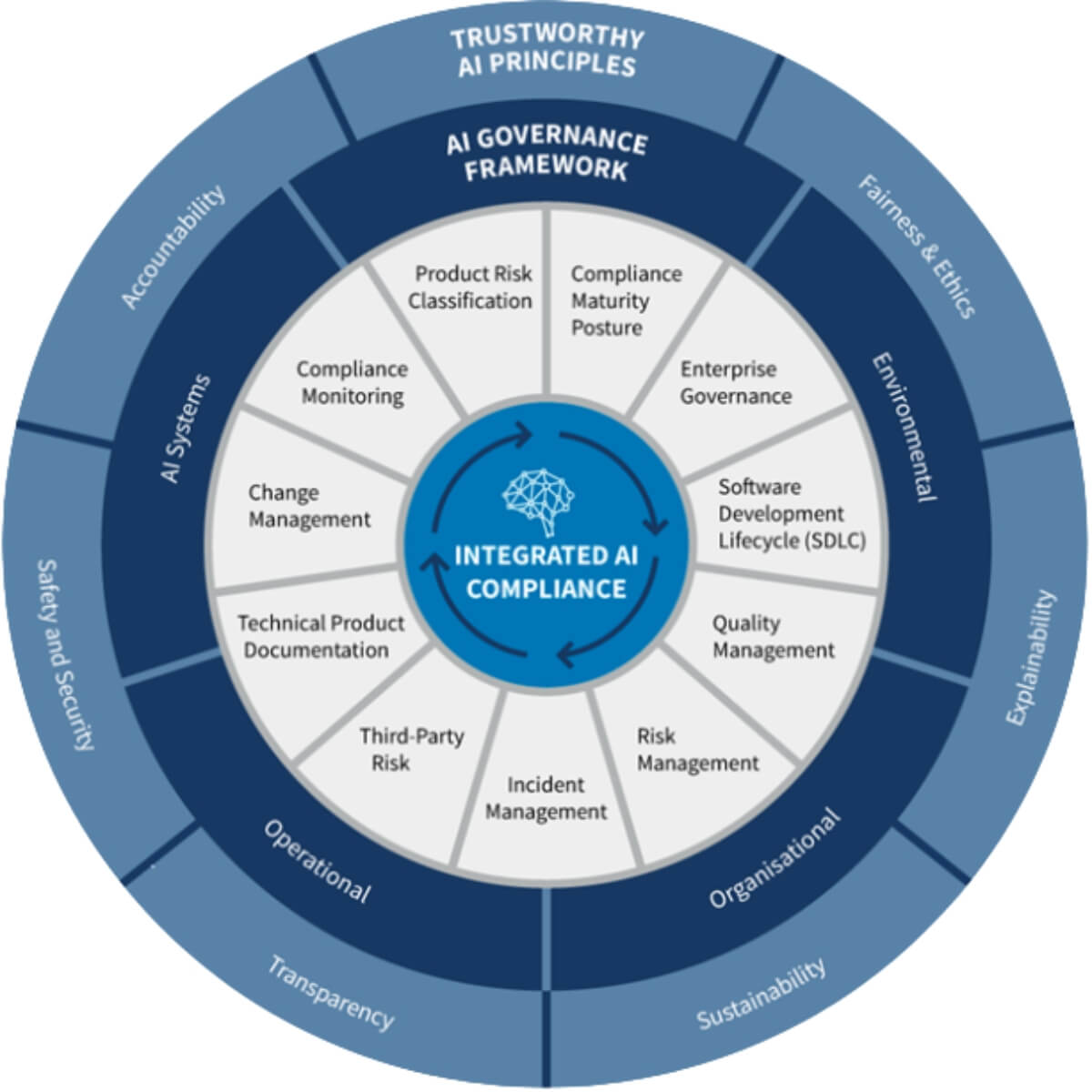
Key findings
RESOURCES
Explore Expert Insights and Resources for Clinical Excellence
Webinars
AI is everywhere these days — but it’s also confusing. What exactly is it? Does it actually work? Why do some companies restrict its use? Are all “AI” tools created equal? And how do generative and agentic AI differ? Join Clinical Maestro’s live webinar to cut through the noise and explore what’s real vs. hype in AI for clinical outsourcing and procurement.

Blogs
Strategikon launches Clinical Maestro® 5.0 with Clinical Maestro AI — transforming clinical outsourcing. Learn how sponsors and CROs benefit from vendor intelligence, rate card compliance, change order simplification, and cost transparency.

Case Studies
Faced with inefficiencies, compliance risks, and fragmented communication, a biopharma company dramatically enhanced its vendor governance by implementing VISION!
DEMO
Request a demo
Discover how Strategikon’s advanced solutions can streamline your clinical trial operations. Request a personalized demo to explore how our tools transform budgeting, vendor management, and outsourcing efficiency for pharma and biotech.