When?
Start:
May 24, 2022
12pm
End:
May 24, 2022
1pm
Speakers
Bruno Gagnon
Senior Vice President, Development Operations at Eidos Therapeutics
Anca Copaescu
CEO of Strategikon
Planning, budgeting, and outsourcing clinical trials are key elements in the life cycle of a biotech company. There are so many considerations: study or program level scenario planning is instrumental to sharpening clinical execution plans; R&D pipeline cost estimates are required for fund raising, CFO reporting, or R&D partnership discussions. Each asset is precious, so selecting the right CRO/clinical service provider and performing operational and budget-level due diligence is mission critical. All these aspects of clinical operations are common to biopharmaceutical companies, but the biotechs have additional challenges; they are usually short staffed, moving at light speed, and lack the clinical outsourcing infrastructure that larger companies have built over many years of running trials. Learn:
- How to overcome common challenges in clinical study planning and budgeting
- Methodologies for effectively conducting CRO proposal due diligence and getting over analysis hurdle
- Reviewing/changing your approach and your process
Hear insights from industry leader and respected subject matter expert- Bruno Gagnon, Senior Vice President, Development Operations at Eidos Therapeutics. Anca Copaescu, CEO of Strategikon will be talking with Bruno to discuss his experiences and how biotechs can embrace new perspectives in clinical outsourcing and will provide know how into challenges and solutions to help support your organization.
Strengthen your processes and increase data-driven decision-making for faster study start-ups.
When?
Start:
May 24, 2022
12pm
End:
May 24, 2022
1pm
Speakers
Bruno Gagnon
Senior Vice President, Development Operations at Eidos Therapeutics
Anca Copaescu
CEO of Strategikon
Strengthen your processes and increase data-driven decision-making for faster study start-ups.
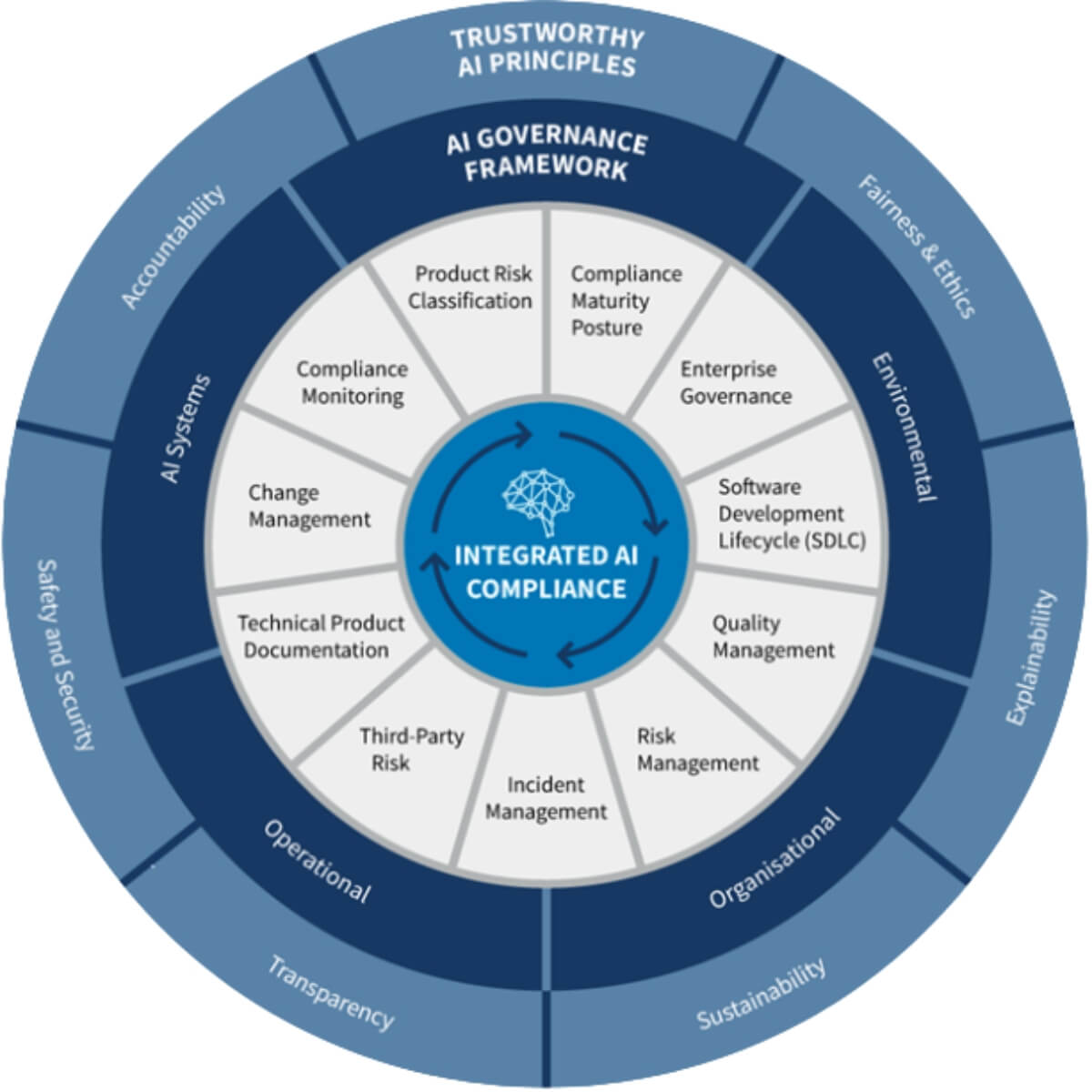
RESOURCES
Explore Expert Insights and Resources for Clinical Excellence
Webinars
AI is everywhere these days — but it’s also confusing. What exactly is it? Does it actually work? Why do some companies restrict its use? Are all “AI” tools created equal? And how do generative and agentic AI differ? Join Clinical Maestro’s live webinar to cut through the noise and explore what’s real vs. hype in AI for clinical outsourcing and procurement.

Blogs
Strategikon launches Clinical Maestro® 5.0 with Clinical Maestro AI — transforming clinical outsourcing. Learn how sponsors and CROs benefit from vendor intelligence, rate card compliance, change order simplification, and cost transparency.

Case Studies
Faced with inefficiencies, compliance risks, and fragmented communication, a biopharma company dramatically enhanced its vendor governance by implementing VISION!
DEMO
Request a demo
Discover how Strategikon’s advanced solutions can streamline your clinical trial operations. Request a personalized demo to explore how our tools transform budgeting, vendor management, and outsourcing efficiency for pharma and biotech.