When?
Start:
October 14, 2025
End:
October 15, 2025
Where?
Barcelona, Spain, at the InterContinental Barcelona (Fira Center)
Details
Event Category:
Conference
This event has passed.
Join Strategikon at SCOPE Europe 2025 in Barcelona to talk about tackling the inefficiency in clinical outsourcing and vendor management.
Strategikon at SCOPE Europe Summit 2025
October 14-15, 2025
We’re Heading to SCOPE Europe
This October, Strategikon will join the SCOPE Europe Summit for Clinical Operations Executives in Barcelona. Our CEO, Anca Copaescu and our EVP Global Business Development, Axel Stael von Holstein will be there — available for one-on-one meetings to talk about smarter outsourcing, optimized vendor relationships, budgeting accuracy, and the operational challenges facing clinical research today.
Let’s Talk About What Matters
At Strategikon, we’ve been working with Sponsors-large and medium biopharma companies and CROs from US, Europe and Asia to tackle the toughest parts of clinical trials operations. If you’ll be at SCOPE, here are some of the topics we’d love to explore together:
Tackling Inefficiency in Clinical Outsourcing
Clinical outsourcing is one of the most complex and critical processes in drug development—60% of all trial activities now rely on it. Every study involves dozens of vendors and countless outsourcing activities, creating a scope of work that quickly becomes overwhelming and hard to manage. Traditional tools just can’t keep up, resulting in “spreadsheet chaos”: tangled processes, wasted resources, and mounting frustration across clinical teams.
At Strategikon, the mission is clear: clinical outsourcing should—and can—be much better. It’s time to save the industry from waste and bottlenecks by simplifying processes, increasing transparency, and speeding up operations with technology purpose-built for clinical research.
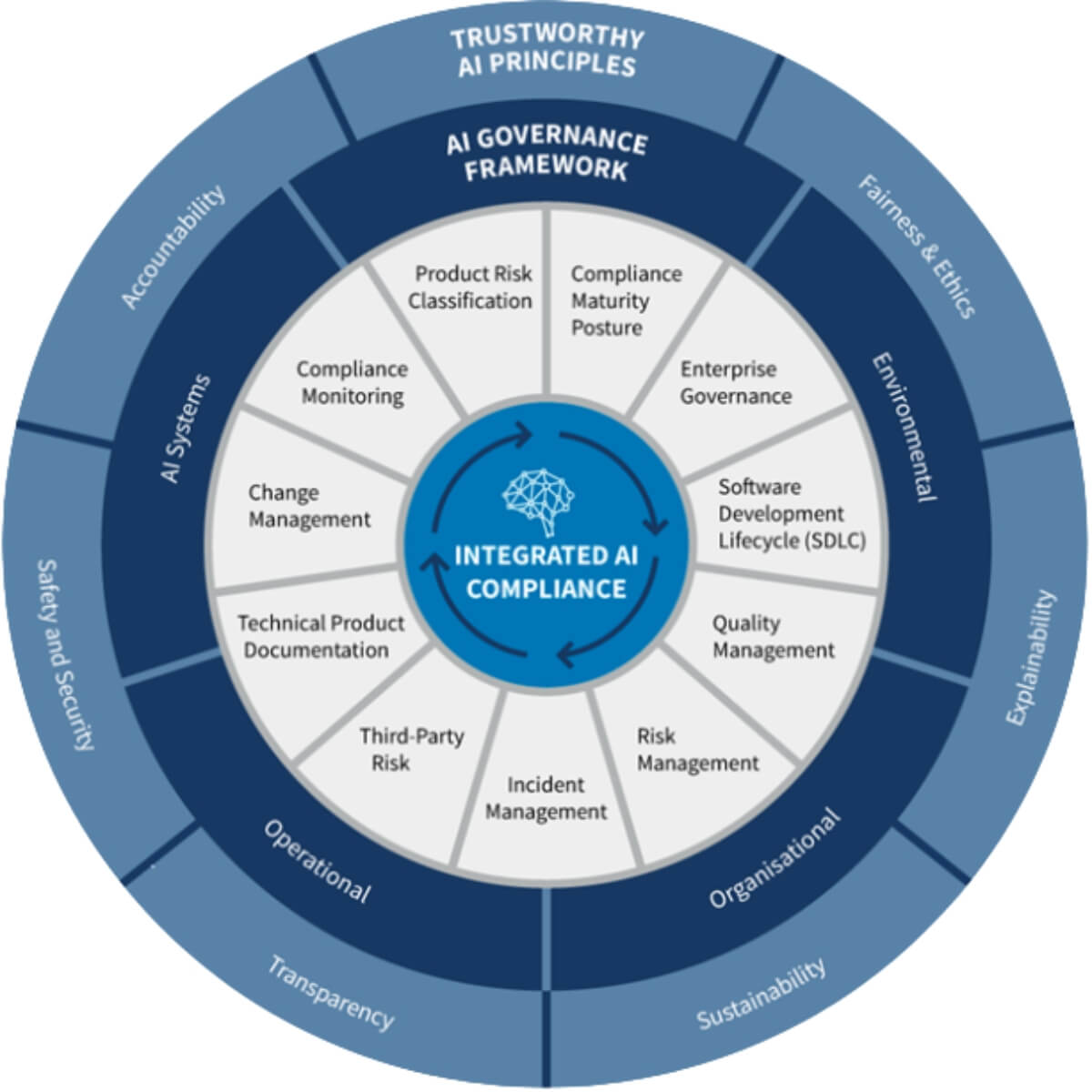
Clinical Maestro: The Orchestration Platform
Strategikon built Clinical Maestro: the industry’s first end-to-end platform for orchestrating clinical outsourcing. What sets it apart?
- A purpose-built workflow layer designed by expert practitioners from both sponsor and CRO sectors, mapping real-world processes and expectations right into the system.
- A structured data layer, where templates, rate cards, and bid grids are digitized—making information not only accessible, but instantly usable and comparable.
- An intelligence layer powered by AI that delivers natural language interaction, analytics, and smart data extraction, giving users actionable insights in a highly unstructured ecosystem.
Revolutionizing the Outsourcing Manager’s Experience
- No more tediously assembling RFPs from fragmented sources.
- Effortlessly launching new sourcing events within minutes using built-in templates, data from previous events, and industry best practices—all through a conversational interface.
- Instantly comparing proposals side-by-side to identify both qualitative and quantitative differences, producing executive summaries automatically.
- Cross-referencing vendor management data and extracting key insights without jumping between systems—she can simply ask the platform for performance trends, risk alerts, or governance details at any moment.
Impact: Measurable Acceleration and Efficiency
Across the client base, Strategikon sees Clinical Maestro delivering 5-10x gains in speed and efficiency for every major stage of the outsourcing process. The result: accelerated trials, faster delivery of medicines to patients, and significant reductions in costly redundancies. The commitment is shared industry-wide—to help teams operate smarter, focus on science, and drive innovation forward with technology.
Why It Matters
SCOPE Europe is where the clinical operations community gathers to share insights and practical solutions. For Strategikon, it’s the perfect place to exchange ideas on:
- How outsourcing clinical trials can be re-designed for efficiency and transparency.
- Why the vendor management process in clinical research is evolving, and how to keep up.
- Smarter ways to handle clinical trial budgeting and forecasting in a changing landscape.
- What clinical trial contract management should look like in practice, not just on paper.
- How clinical vendor management supports better partnerships and outcomes.
- Where clinical trial proposal management can save valuable time across the industry.
Meet Us in Barcelona
If you’d like to connect about outsourcing strategies, vendor oversight, budgeting, contracting, or proposals, drop us a note at [info@strategikon.com] and let’s find a time.
We’re looking forward to meaningful conversations at SCOPE Europe — and to working together on making clinical research simpler, smarter, and more effective.
Strengthen your processes and increase data-driven decision-making for faster study start-ups.
Strengthen your processes and increase data-driven decision-making for faster study start-ups.
When?
Start:
October 14, 2025
End:
October 15, 2025
Where?
Barcelona, Spain, at the InterContinental Barcelona (Fira Center)
Details
Event Category:
Conference
This event has passed.
RESOURCES
Explore Expert Insights and Resources for Clinical Excellence
Webinars
AI is everywhere these days — but it’s also confusing. What exactly is it? Does it actually work? Why do some companies restrict its use? Are all “AI” tools created equal? And how do generative and agentic AI differ? Join Clinical Maestro’s live webinar to cut through the noise and explore what’s real vs. hype in AI for clinical outsourcing and procurement.

Blogs
Strategikon launches Clinical Maestro® 5.0 with Clinical Maestro AI — transforming clinical outsourcing. Learn how sponsors and CROs benefit from vendor intelligence, rate card compliance, change order simplification, and cost transparency.

Case Studies
Faced with inefficiencies, compliance risks, and fragmented communication, a biopharma company dramatically enhanced its vendor governance by implementing VISION!
DEMO
Request a demo
Discover how Strategikon’s advanced solutions can streamline your clinical trial operations. Request a personalized demo to explore how our tools transform budgeting, vendor management, and outsourcing efficiency for pharma and biotech.