In this case study
Strengthen your processes and increase data-driven decision-making for faster study start-ups.
Strengthen your processes and increase data-driven decision-making for faster study start-ups.
In this case study
Strengthen your processes and increase data-driven decision-making for faster study start-ups.
RESOURCES
Explore Expert Insights and Resources for Clinical Excellence

Webinars
CRO budgets: complex, time-consuming, and often a black box. But they don’t have to be. Join us for a high-impact session where Leslie Mathews, Anca Copaescu, and Andrei Antonescu walk you through the real-world mechanics of CRO budgeting—from decoding activity grids to pressure-testing your numbers with powerful benchmarks.
Blogs
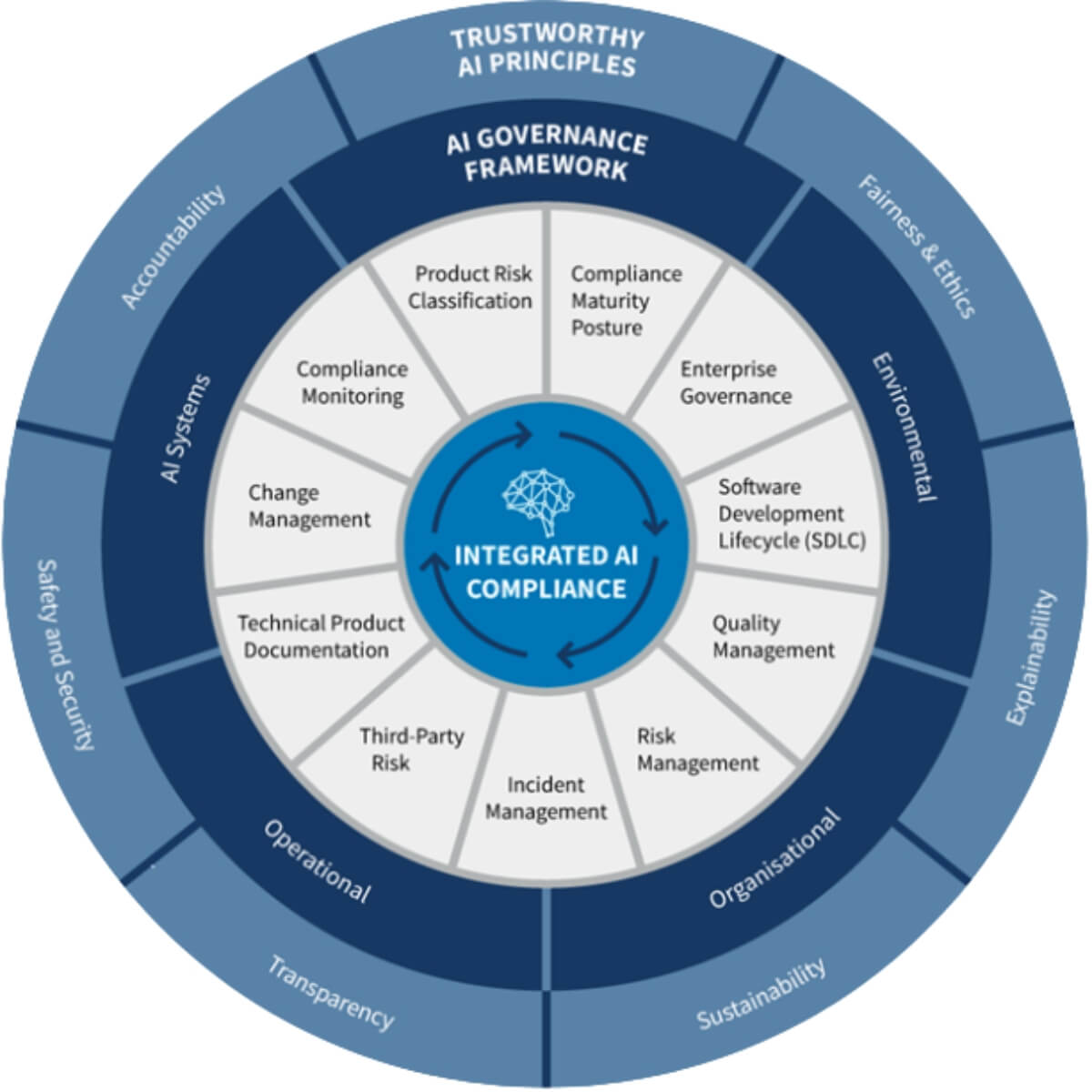
Effective vendor governance is essential to ensure operational success, cost efficiency, and regulatory compliance. Learn more here in our blog!

Case Studies
Faced with inefficiencies, compliance risks, and fragmented communication, a biopharma company dramatically enhanced its vendor governance by implementing VISION!
DEMO
Request a demo
Discover how Strategikon’s advanced solutions can streamline your clinical trial operations. Request a personalized demo to explore how our tools transform budgeting, vendor management, and outsourcing efficiency for pharma and biotech.