Industry
Biopharmaceutical
Products used
Clinical Maestro PORTFOLIO™
0%
Variance
between the awarded CRO budget and the Clinical Maestro calculated budget
2.25%
Variance
between the awarded CRO budget and the Clinical Maestro mid-intensity calculated budget
+/-1.5%
Therapeutic Area Accuracy
significantly increases with refinements to Clinical Maestro algorithms
In this case study
Strengthen your processes and increase data-driven decision-making for faster study start-ups.
Strengthen your processes and increase data-driven decision-making for faster study start-ups.
In this case study
Strengthen your processes and increase data-driven decision-making for faster study start-ups.
RESOURCES
Explore Expert Insights and Resources for Clinical Excellence
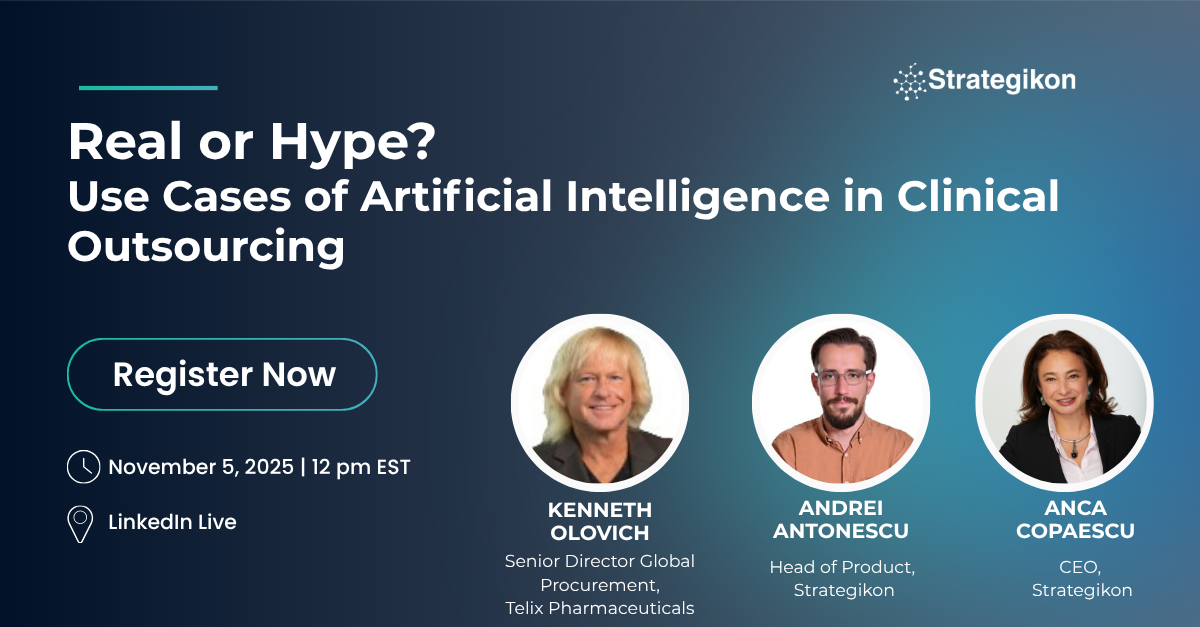
Webinars
AI is everywhere these days — but it’s also confusing. What exactly is it? Does it actually work? Why do some companies restrict its use? Are all “AI” tools created equal? And how do generative and agentic AI differ? Join Clinical Maestro’s live webinar to cut through the noise and explore what’s real vs. hype in AI for clinical outsourcing and procurement.

Blogs
Strategikon launches Clinical Maestro® 5.0 with Clinical Maestro AI — transforming clinical outsourcing. Learn how sponsors and CROs benefit from vendor intelligence, rate card compliance, change order simplification, and cost transparency.

Case Studies
Faced with inefficiencies, compliance risks, and fragmented communication, a biopharma company dramatically enhanced its vendor governance by implementing VISION!
DEMO
Request a demo
Discover how Strategikon’s advanced solutions can streamline your clinical trial operations. Request a personalized demo to explore how our tools transform budgeting, vendor management, and outsourcing efficiency for pharma and biotech.