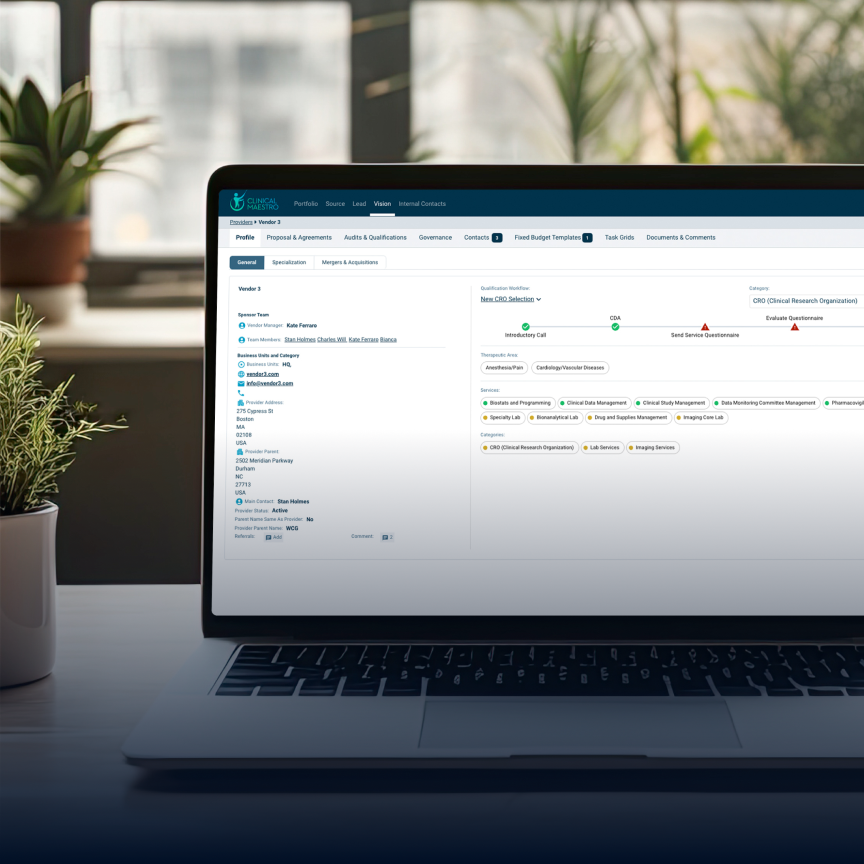
OVERVIEW
Empowering small biotechs with accurate, efficient budgeting and planning
With Clinical Maestro’s advanced budgeting and scenario modeling tools, companies of any size can create accurate study plans and budgets with ease – in only a few hours. Gain the assurance of data-backed analyses and flexibility to optimize your protocol. If you don’t have the resources, Clinical Maestro’s services are always available to support lean organizations from consulting to determine the best path forward, to helping you implement the technology within your organization to running the technology for you with managed services.
MARKET PROBLEM
Overcoming Planning, Budgeting, and Vendor Management Challenges for Small Biopharma Success
Small biopharma companies face operational challenges due to tight budgets, limited planning and budgeting experience, lack of reference benchmarks, and resource constraints, all of which make accurate forecasting and vendor transparency difficult, impacting trial success.

Limited Time, Budgeting Resources and Expertise
Overreliance on Excel and limited resources strain operations, reducing productivity, increasing errors, and diverting time from strategic activities essential for trial success and investor reporting.
Insufficient Sourcing Expertise and Overreliance on CROs
Small biopharma companies often lack in-house sourcing experience, leading to an overreliance on CROs for guidance. This dependency can drive up costs, reduce control over trial quality, and limit operational flexibility.
Ineffective Forecasting and Scenario Planning
Without precise forecasting tools, predicting trial costs and outcomes is challenging, resulting in financial unpredictability that complicates planning and disrupts project stability.
Limited Vendor Transparency and Cost Verification
Manual processes make it difficult for small biopharma to gain clear visibility into vendor activities and costs. This limits the ability to verify invoices, track completed work, forecast future activities, and identify out-of-scope expenses, causing frustration and inefficiencies
BENEFITS
Streamline Trial Planning and Budgeting with Clinical Maestro for Biopharma
Clinical Maestro empowers small biopharma with efficient budgeting, vendor management, and contract oversight, providing data-driven solutions to optimize trial planning, reduce costs, and enhance operational control.

Fast, Accurate Budgeting and Planning
Data-driven budgeting and forecasting tools help small biopharma create precise financial plans with minimal resources reducing planning time and ensuring data-backed decisions to avoid unplanned costs and delays.

Faster Due Diligence and Collaboration for Simplified Sourcing
Leverage real-time market benchmarks and streamlined sourcing tools to conduct due diligence up to 10x faster, select quality vendors for streamlined decision-making.

Simplified Change Order and Accrual Tracking
Track actuals and accruals to budget with a transparent change order process, efficiently engaging suppliers and optimizing resource use.

Increased Negotiation Power with CROs
Utilize data-driven insights and operational visibility to maximize savings and negotiate more favorable terms with CROs confidently. Track vendor performance with real-time analytics, ensure alignment with study goals and quality standards.
CASE STUDY
Near-Perfect Budget Accuracy Achieved in Phase II CNS Trial

West-coast biotech does well!
2X
Faster from RFP to final budget
4 months
less time to source study compared to traditional tools
$800k
saved by enabling effective negotiations
Clinical Maestro streamlined RFP processes, improved due diligence, and enhanced decision-making.
Clinical Maestro streamlined RFP processes, improved due diligence, and enhanced decision-making.
West-coast biotech does well!
2X
Faster from RFP to final budget
4 months
less time to source study compared to traditional tools
$800k
saved by enabling effective negotiations

Mid-size Biopharma company
0%
variance achieved between CRO bids and Clinical Maestro-generated budgets for Phase I vaccine
2.25%
variance delivered by Clinical Maestro’s advanced algorithms for Phase II
+/-1.5%
accuracy achieved through refinements in cost models
Clinical Maestro enabled a biotech to deliver precise vaccine trial budgets, achieving great results with refined cost models
Clinical Maestro enabled a biotech to deliver precise vaccine trial budgets, achieving great results with refined cost models
Mid-size Biopharma company
0%
variance achieved between CRO bids and Clinical Maestro-generated budgets for Phase I vaccine
2.25%
variance delivered by Clinical Maestro’s advanced algorithms for Phase II
+/-1.5%
accuracy achieved through refinements in cost models

Leading Japanese Pharma Drives Budget Accuracy for Complex Oncology Study
3 hours
to build budgets and scenarios
12%
minimal variance between CRO bids and Clinical Maestro
4 scenarios
optimized for cost efficiency
PORTFOLIO enables accurate budgeting for high-complexity trials, reducing variances, enhancing efficiency, and optimizing CRO negotiations.
PORTFOLIO enables accurate budgeting for high-complexity trials, reducing variances, enhancing efficiency, and optimizing CRO negotiations.
Leading Japanese Pharma Drives Budget Accuracy for Complex Oncology Study
3 hours
to build budgets and scenarios
12%
minimal variance between CRO bids and Clinical Maestro
4 scenarios
optimized for cost efficiency
RESOURCES
Explore Expert Insights and Resources for Clinical Excellence
Webinars
AI is everywhere these days — but it’s also confusing. What exactly is it? Does it actually work? Why do some companies restrict its use? Are all “AI” tools created equal? And how do generative and agentic AI differ? Join Clinical Maestro’s live webinar to cut through the noise and explore what’s real vs. hype in AI for clinical outsourcing and procurement.

Blogs
Strategikon launches Clinical Maestro® 5.0 with Clinical Maestro AI — transforming clinical outsourcing. Learn how sponsors and CROs benefit from vendor intelligence, rate card compliance, change order simplification, and cost transparency.

Case Studies
Faced with inefficiencies, compliance risks, and fragmented communication, a biopharma company dramatically enhanced its vendor governance by implementing VISION!
DEMO
Request a demo
Discover how Strategikon’s advanced solutions can streamline your clinical trial operations. Request a personalized demo to explore how our tools transform budgeting, vendor management, and outsourcing efficiency for pharma and biotech.