Simplify and Optimize R&D Procurement Processes
Streamline vendor selection, manage RFIs and RFPs, and automate contracts with Clinical Maestro® for efficient, transparent procurement.

OUR SOLUTIONS
Streamline Vendor Management and Clinical Outsourcing Efficiency
Optimize vendor selection, contract management, and trial processes with Clinical Maestro®’s solutions. Centralized tools improve compliance, accelerate timelines, reduce costs, and enhance decision-making for efficient, transparent clinical trial operations.
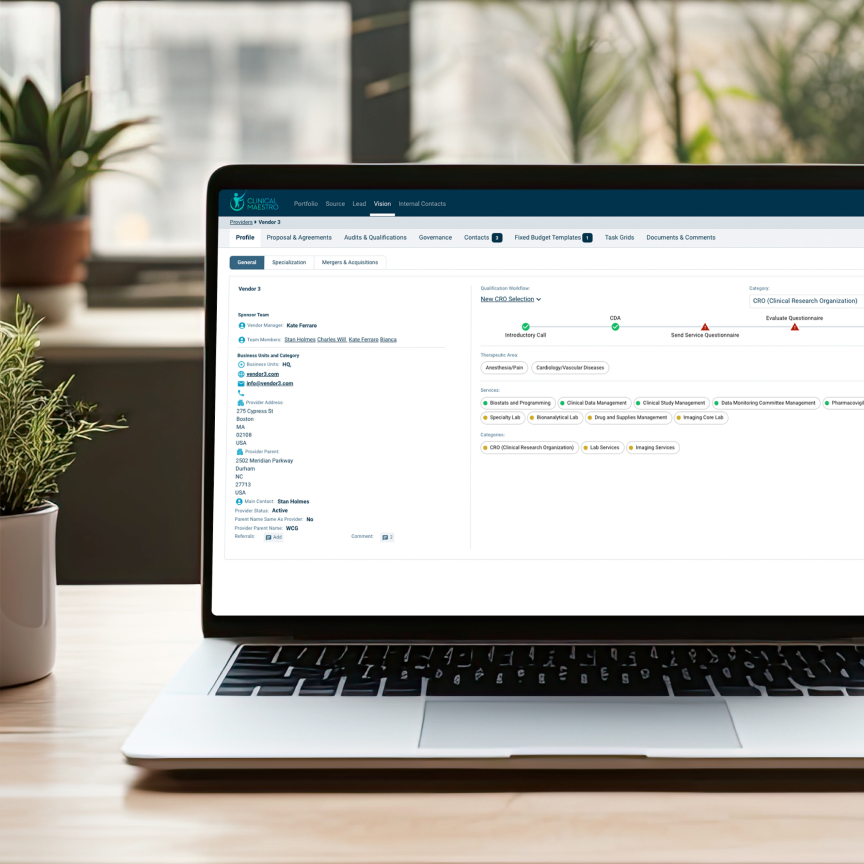
Qualify Service Providers
A growing pharmaceutical company faced fragmented vendor qualification processes, leading to delays, inconsistent vendor selection, and costly inefficiencies. By adopting Strategikon’s VISION solution, they streamlined vendor management, improved compliance, and accelerated trial start-up timelines.
![]()
Centralized Vendor Profiles
Access real-time data on vendor qualifications, capabilities, and compliance for faster, more reliable selection.
![]()
Enhanced Performance Tracking
Track vendor performance metrics across trials, ensuring consistent, high-quality service and future reliability.
![]()
Reduced Qualification Time
Shorten vendor qualification by 40%, allowing trials to begin sooner and stay on schedule.
Manage RFIs and RFPs
Biopharma companies face disorganized RFP and RFI processes, leading to inefficiencies, miscommunication, and delays in vendor selection. By adopting Clinical Maestro® SOURCE, they streamlined workflows, enhanced transparency, and accelerated decision-making, saving time and reducing outsourcing costs.
![]()
Centralized Sourcing Activities
Standardized RFPs and RFIs streamline workflows, ensuring consistent vendor submissions and efficient management.
![]()
Real-Time Bid Comparisons
Easily analyze proposals side by side, assessing costs, timelines, and service offerings with clarity.
![]()
Accelerated Decision Process
Reduce response delays by 30%, accelerating the review process and improving study start-up timelines by 20%.


Manage Change Orders
Managing change orders in clinical trials was a challenge for R&D procurement teams due to outdated manual processes. Clinical Maestro® SOURCE and LEAD solutions streamline your workflows, increase data accuracy, and improve visibility. The strategic combination reduces delays and strengthens vendor relationships.
![]()
Automated Change Tracking
Eliminates manual processes, providing real-time visibility into out-of-scope activities and budget updates.
![]()
Enhanced Budget Accuracy
Reduces financial misreporting risk by 95%, ensuring precise forecasting and proactive budget management.
![]()
Streamlined Vendor Negotiations
Improves communication and transparency, enabling faster contract adjustments and stronger adherence to timelines and budgets.
Generate Contracts
Pharmaceutical companies faced delays and inefficiencies in contract generation due to manual processes. By implementing Clinical Maestro® SOURCE, they automated data entry, reduced errors, and accelerated review times, enabling faster contract finalization and improved trial timelines.
![]()
Automated Data Population
Dynamically populates contracts with proposal data, eliminating manual entry and reducing errors.
![]()
Faster Review Processes
Streamlined workflows and integrations to your preferred CLM solution allow stakeholders to review contracts online, minimizing bottlenecks and delays.
![]()
Improved Productivity
Boosts efficiency by over 90%, ensuring quicker contract execution and timely clinical trial initiation.


Bid Grid Mapping
Pharmaceutical companies struggle with complex, inconsistent CRO bid comparisons, leading to inefficiencies, delays, and cost overruns. By implementing Clinical Maestro® SOURCE, you can streamline bid management, improve transparency, and reduce errors, achieving better vendor negotiations and cost savings.
![]()
Standardized Bid Comparisons
Automates bid normalization into templates, enabling accurate side-by-side evaluations of cost drivers and resources.
![]()
Faster Evaluation Processes
Reduces bid evaluation time by 40%, accelerating decision-making and keeping trials on schedule.
![]()
Cost Savings
Enhances negotiation capabilities, saving over 10% on trial budgets for reinvestment in R&D initiatives.
RESOURCES
Explore Expert Insights and Resources for Clinical Excellence
Webinars
AI is everywhere these days — but it’s also confusing. What exactly is it? Does it actually work? Why do some companies restrict its use? Are all “AI” tools created equal? And how do generative and agentic AI differ? Join Clinical Maestro’s live webinar to cut through the noise and explore what’s real vs. hype in AI for clinical outsourcing and procurement.

Blogs
Strategikon launches Clinical Maestro® 5.0 with Clinical Maestro AI — transforming clinical outsourcing. Learn how sponsors and CROs benefit from vendor intelligence, rate card compliance, change order simplification, and cost transparency.

Case Studies
Faced with inefficiencies, compliance risks, and fragmented communication, a biopharma company dramatically enhanced its vendor governance by implementing VISION!
DEMO
Request a demo
Discover how Strategikon’s advanced solutions can streamline your clinical trial operations. Request a personalized demo to explore how our tools transform budgeting, vendor management, and outsourcing efficiency for pharma and biotech.



















